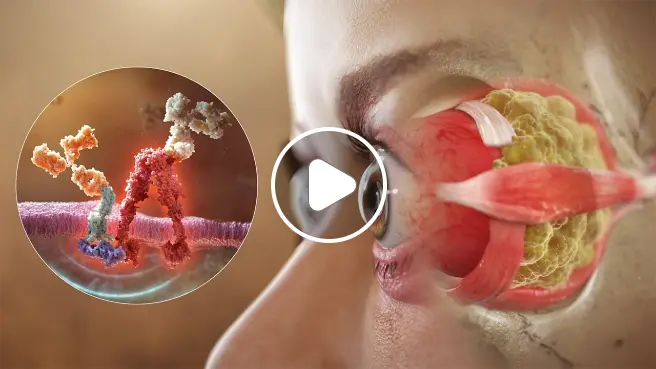
THYROID EYE DISEASE (TED) PATHOPHYSIOLOGY AND IGF-1R/TSHR SIGNALING
IGF-1R activation on orbital fibroblasts is believed to control the pathophysiology of Thyroid Eye Disease (TED) throughout the course of disease1
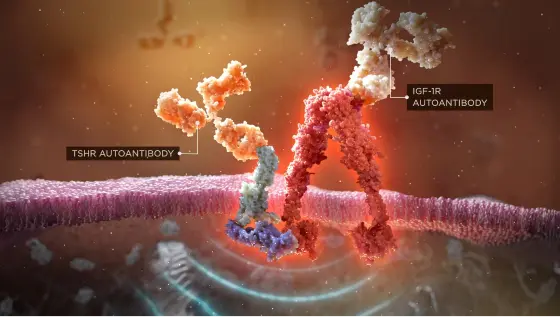
Autoantibodies initiate the IGF-1R/TSHR signaling complex, and this activates orbital fibroblasts1-3

Once stimulated, orbital fibroblasts cause inflammation and the expansion of muscle and fat tissue behind the eye3,4
TED DAMAGE BEGINS BEHIND THE EYE4
Orbital fibroblasts become targets
Before symptoms such as erythema or proptosis are visible, TED autoantibodies target orbital fibroblasts, the specialized cells responsible for tissue and cell repair, via the IGF-1R/TSHR signaling complex.1-3 The resulting inflammatory cascade causes muscle tissue within the orbit to swell. Adipose tissue at the back of the orbit also expands. These tissue changes within the rigid, bony orbital space force the eye forward and exert a dangerous amount of pressure on the optic nerve.3-5
Imaging can reveal the damage
MRI studies can provide objective confirmation of the substantial inflammatory changes in and around the orbit and show the involvement of individual muscles, even early in the course of disease.6 In one study, orbital MRI revealed extraocular muscular swelling in 70% of patients who had Graves’ disease but no visible signs of TED.7,8
The damage can be progressive
The swelling of muscle and expansion of orbital fat seen on MRI images can cause ocular tenderness, pain, and pressure. As TED progresses, the increased intraorbital volume puts pressure on the eye and optic nerve as more pronounced signs begin to appear, such as proptosis/exophthalmos, vision changes, and signs of corneal exposure.5
RISK FACTORS AND PREVALENCE OF TED



AGE AND GENDER
Older age is a nonmodifiable risk factor for the development and severity of TED. Additionally, TED occurs 5 times more often in women than men—though men often have a more severe form of TED.4,9,10
CIGARETTE SMOKING
This is the strongest modifiable risk factor for TED. Smokers are nearly 8 times more likely to develop TED than nonsmokers.4
THYROID DYSFUNCTION AND RADIOACTIVE IODINE TREATMENT
These are among the modifiable risk factors for TED.9
The comorbidities associated with TED
A retrospective study of data from 667 US patients with TED suggests that patients with this disease often have additional thyroidal and nonthyroidal comorbidities. Patients who have severe TED have a potentially greater likelihood of having both types.11 The majority had more than 1 nonthyroidal comorbidity, with 44% reporting at least 2 nonthyroidal comorbidities.11
The most common nonthyroidal comorbidities were:
- Hypertension
- Anxiety
- Depression
- Type 2 diabetes
- Nonthyroidal autoimmune diseases
In the study, patients with severe TED (13%) had a significantly greater number of nonthyroidal comorbidities than mild patients (1.8 vs 1.3; P<0.001) despite similar age, ethnicity, and gender distribution. Severe patients were also more likely to have nonthyroidal autoimmune disease (22% vs 11%), hyperglycemia (15% vs 6%), skin complications (6% vs 1%), and liver disease or complications (5% vs 1%) [P<0.023].11
THE IMPACT AND CONSEQUENCES OF TED
The consequences of TED are potentially debilitating.12 While TED may cause lifelong ocular discomfort, diplopia, proptosis, and facial disfigurement, in few patients, TED can lead to permanent vision loss.1
TED can have a debilitating impact on daily life, negatively affecting employment, relationships, and self-confidence, frequently leading patients to withdraw from social interactions.13-15
Early intervention is important. Prompt consultation with a TED Specialist may help outcomes for patients with symptoms of TED.16 A TED Specialist can perform a comprehensive TED eye exam and order an MRI to look for swelling or fat around the eyes, as this can be an early sign of TED.4,8
DISEASE ACTIVITY AND SEVERITY
TED is a lifelong, progressive, debilitating autoimmune disease that can present with varying degrees of disease activity including orbital inflammation and tissue expansion.17 TED patients may experience quiescence of some inflammatory symptoms. Despite some symptoms appearing to subside or disappear, proptosis and diplopia can remain, and other inflammatory signs can reactivate or flare over time.9,18 The morbidity burden can be considerable, with a marked impact on quality of life, mental health, and employment rate.9,19,20
TED was once thought to have two distinct phases of active/inflammatory symptoms followed by inactive/stable symptoms. Evidence now suggests that the same underlying disease pathophysiology may drive TED through the course of disease.1,21,22
Referring your patients to a TED Specialist can be an important step in helping to reduce the burden of this progressive disease, regardless of disease progression or activity.9,19
IGF-1R, insulin-like growth factor-1 receptor; MRI, magnetic resonance imaging; THSR, thyroid-stimulating hormone receptor.
Next page > Psychological Impact of TED
REFERENCES:
- Wang Y, Patel A, Douglas RS. Ther Clin Risk Manag. 2019;15:1305-1318.
- Krieger CC, Boutin A, Jang D, et al. Arrestin-β-1 physically scaffolds TSH and IGF1 receptors to enable crosstalk. Endocrinol. 2019;160(6):1468-1479.
- Dik WA, Virakul S, van Steensel L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Exp Eye Res. 2016;142:83-91.
- Bahn RS. Graves’ ophthalmopathy. N Engl J Med. 2010;362(8):726-738.
- Barrio-Barrio J, Sabater AL, Bonet-Farriol E, Velázquez-Villoria Á, Galofré JC. Graves’ ophthalmopathy: VISA versus EUGOGO classification, assessment, and management. J Ophthalmol. 2015;2015:249125.
- Kilicarslan R, Alkan A, Ilhan MM, Yetis H, Aralasmak A, Tasan E. Graves’ ophthalmopathy: the role of diffusion-weighted imaging in detecting involvement of extraocular muscles in early period of disease. Br J Radiol. 2015;88(1047):20140677.
- Villadolid MC, Yokoyama N, Isumi M, et al. Untreated Graves’ disease patients without clinical ophthalmopathy demonstrate a high frequency of extraocular muscle (EOM) enlargement by magnetic resonance. J Clin Endocrinol Metab. 1995;80(9):2830-2833.
- Smith TJ, Hegedüs L. Graves’ disease. N Engl J Med. 2016;375(16):1552-1665.
- Burch HB, Perros P, Bednarczuk T, et al. Management of Thyroid Eye Disease: a consensus statement by the American Thyroid Association and the European Thyroid Association. Thyroid. 2022;32(12):1-32.
- Thyroid Eye Disease. National Organization for Rare Disorders. 2020. Accessed December 8, 2022. https://rarediseases.org/rare-diseases/thyroid-eye-disease
- Campbell P. ATA study details risk factors, comorbidities associated with severe Thyroid Eye Disease. Endocrinology Network. October 7, 2021. Accessed January 17, 2023. https://www.endocrinologynetwork.com/view/ata-study-details-risk-factors-comorbidities-associated-with-severe-thyroid-eye-disease
- Wu CY, Niziol LM, Musch DC, Kahana A. Thyroid-related orbital decompression surgery: a multivariate analysis of risk factors and outcomes. Ophthal Plast Reconstr Surg. 2017;33(3):189-195.
- Kahaly GJ, Petrak F, Hardt J, Pitz S, Egle UT. Psychosocial morbidity of Graves’ orbitopathy. Clin Endocrinol (Oxf). 2005;63(4):395-402.
- Estcourt S, Vaidya B, Quinn A, Shepherd M. The impact of Thyroid Eye Disease upon patients’ wellbeing: a qualitative analysis. Clin Endocrinol (Oxf). 2008;68(4):635-9.
- Terwee C, Wakelkamp I, Tan S, Dekker F, Prummel MF, Wiersinga W. Long-term effects of Graves’ ophthalmopathy on health-related quality of life. Eur J Endocrinol. 2002;146(6):751-757.
- Mitchell AL, Goss L, Mathiopoulou L, et al. Diagnosis of Graves’ orbitopathy (DiaGO): results of a pilot study to assess the utility of an office tool for practicing endocrinologists. J Clin Endocrin Metab. E2015;100(3):E458-462.
- Park JJ, Sullivan TJ, Mortimer RH, Wagenaar M, Perry-Keene DA. Assessing quality of life in Australian patients with Graves’ ophthalmopathy. Br J Ophthalmol. 2004;88(1):75-78.
- Liaboe CA, Clark TJ, Simmons BA, Carter K, Shriver EM. Thyroid Eye Disease: an introductory tutorial and overview of disease. April 23, 2020. Accessed on January 24, 2023. https://eyerounds.org/tutorials/thyroid-eye-disease/index.htm
- Wang Y, Padnick-Silver L, Francis-Sedlak M, Holt RJ, Foley C, Douglas RS. Inflammatory and noninflammatory Thyroid Eye Disease: comparison of disease signs, symptoms, and quality of life in patients in the United States. Endocrin Pract. 2022;28(9):842-846.
- Ponto KA, Pitz S, Pfeiffer N, Hommel G, Weber MM, Kahaly GJ. Quality of life and occupational disability in endocrine orbitopathy. Dtsch Arztebl Int. 2009;106(17):283-289.
- Ugradar S, Hoang SA, Ward C, et al. Whole genome transcriptome comparison of acute and chronic Thyroid Eye Disease: emergence of a molecular signature. Presented at: American Academy of Ophthalmology; September 30-October 3, 2022.
- Ugradar S, Kang J, Kossler AL, et al. Teprotumumab for the treatment of chronic Thyroid Eye Disease. Eye (Lond). 2022;36(8):1553-1559.